Can Retatrutide Treat MASLD? Evidence from Recent Clinical Trials
Retatrutide demonstrates significant liver fat reduction in MASLD Phase 2 trials. Review the evidence, safety profile, and what these results mean for metabolic liver disease treatment.
Introduction
Metabolic dysfunction-associated steatotic liver disease (MASLD) affects approximately 38% of adults globally, making it the most common chronic liver condition worldwide. Despite this prevalence, effective pharmaceutical treatments have been notably absent. Retatrutide, a triple-receptor agonist, may change this landscape based on recent Phase 2 clinical data.
MASLD: Scope and Consequences
MASLD represents a spectrum of liver disease driven by metabolic dysfunction. The condition begins with hepatic steatosis, fat accumulation in liver cells, and can progress to metabolic dysfunction-associated steatohepatitis (MASH), where inflammation and cellular damage occur. Approximately 20-30% of MASLD cases advance to MASH, which can lead to fibrosis, cirrhosis, and hepatocellular carcinoma.
Current projections indicate a 56% increase in MASLD cases over the next decade, driven by rising obesity and metabolic syndrome rates. This trajectory has significant healthcare implications: increased hospitalizations, higher transplant demand, and substantial economic burden from both direct medical costs and lost productivity.
Why Existing Treatments Are Insufficient
Standard management centers on lifestyle modification, 7-10% weight loss can improve liver histology. However, most patients cannot achieve or maintain this level of weight reduction through diet and exercise alone.
Pharmaceutical options remain limited:
- Vitamin E: Shows benefit in non-diabetic patients but carries long-term safety concerns
- Pioglitazone: Improves liver histology but causes weight gain and other problematic side effects
- Resmetirom: The only FDA-approved medication for MASH with fibrosis, reduces liver fat but doesn't address underlying metabolic dysfunction
The core problem is that MASLD is fundamentally a metabolic disorder manifesting in the liver. Effective treatment requires addressing insulin resistance, dyslipidemia, inflammation, and hormonal regulation of appetite and energy expenditure simultaneously—a challenge for single-target drugs.
Retatrutide's Triple-Agonist Mechanism
Retatrutide simultaneously activates three receptor pathways:
GLP-1 (glucagon-like peptide-1): Reduces appetite, slows gastric emptying, enhances insulin secretion, and improves peripheral glucose uptake.
GIP (glucose-dependent insulinotropic polypeptide): Amplifies insulin sensitivity and appears to directly affect adipose tissue metabolism.
Glucagon: Increases energy expenditure and promotes hepatic fat oxidation.
This combination produces substantial weight loss—often exceeding 20% of body weight—while directly influencing hepatic lipid metabolism. The drug enhances triglyceride breakdown while reducing new fat synthesis in hepatocytes. Critically, retatrutide's effects on liver health appear to exceed what weight loss alone would predict, suggesting direct metabolic benefits beyond caloric deficit.
Phase 2 Clinical Trial Results (2024)
The Phase 2 data demonstrated substantial efficacy in patients with biopsy-confirmed MASH and significant fibrosis:
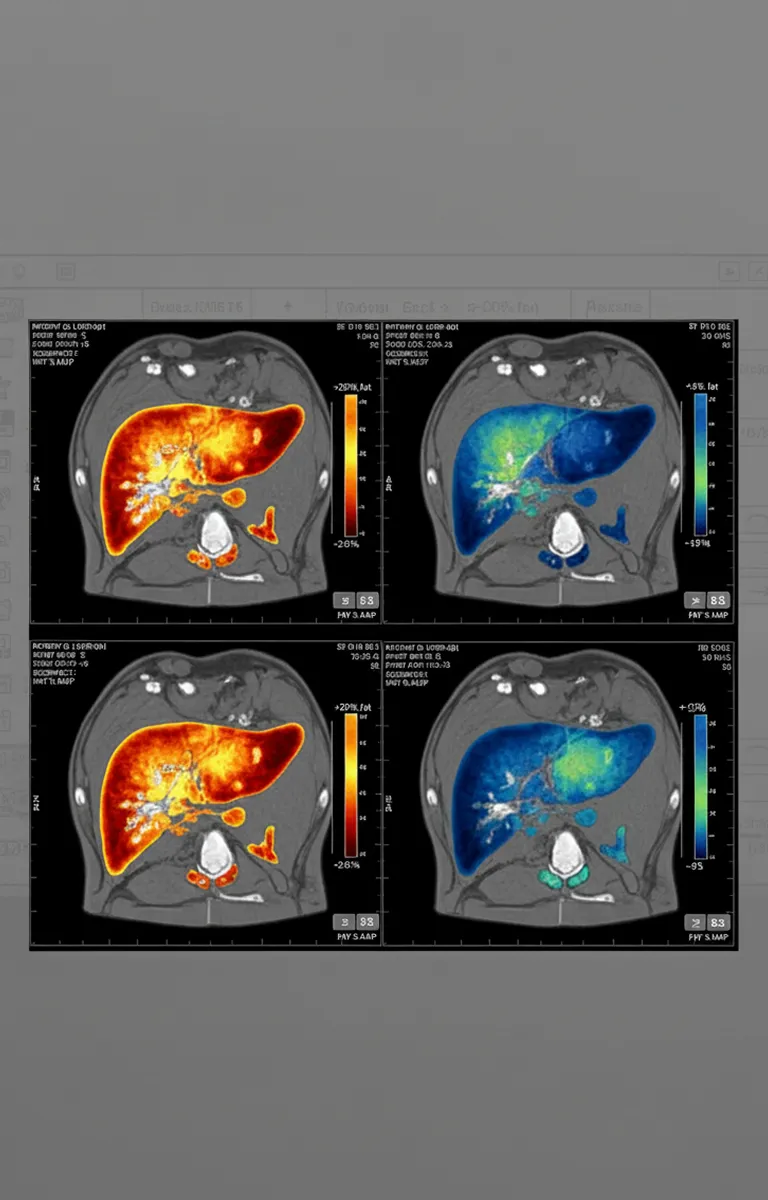
Liver fat reduction: Up to 80% absolute reduction in liver fat content (measured by MRI-PDFF) after 48 weeks
Steatosis resolution: Approximately 86% of patients in the highest dose group achieved liver fat below 5%—the threshold defining steatosis resolution
Histological improvements: Significant rates of both MASH resolution and fibrosis regression on liver biopsy
Metabolic benefits:
- Average weight loss of 24% in highest dose cohorts
- Significant HbA1c reductions in diabetic participants
- Improved lipid profiles (reduced triglycerides, increased HDL)
- Decreased inflammatory biomarkers
Safety profile: Gastrointestinal side effects (nausea, diarrhea, vomiting) were common but generally transient and rarely led to discontinuation. Serious adverse events were comparable between treatment and placebo groups.
Outstanding Questions and Research Priorities
While results are promising, several critical questions require Phase 3 trial data:
Durability: Preliminary evidence suggests some fat reaccumulation after treatment cessation. Is lifelong therapy necessary?
Cardiovascular outcomes: MASLD patients face elevated cardiovascular risk. Does retatrutide's favorable metabolic profile translate to reduced cardiovascular events? Dedicated outcome trials are underway.
Optimal patient selection: Current trials focus on established MASH with fibrosis. Would earlier intervention during simple steatosis prevent progression? How does the risk-benefit profile change in advanced cirrhosis?
Combination strategies: Could retatrutide work synergistically with resmetirom or emerging anti-fibrotic agents? Might lower doses combined with complementary medications provide similar benefits with fewer side effects?
The Multi-Target Drug Paradigm
Retatrutide exemplifies a broader shift in metabolic disease therapeutics. MASH progression requires multiple dysfunctional pathways—insulin resistance, lipotoxicity, oxidative stress, mitochondrial dysfunction, and inflammatory signaling. Single-pathway interventions rarely produce meaningful results.
The pharmaceutical pipeline now includes numerous multi-agonist candidates targeting various receptor combinations. Beyond incretins, researchers are exploring:
- Thyroid hormone receptor modulators
- PPAR nuclear receptor activators
- Anti-inflammatory pathway inhibitors
- Direct anti-fibrotic agents targeting hepatic stellate cells
The optimal approach may involve carefully sequenced combinations, achieving metabolic control and weight loss first, then adding agents that directly address inflammation or fibrosis.
Clinical Implications
If Phase 3 trials confirm Phase 2 findings, retatrutide could become the first medication to produce clinically meaningful improvements in MASLD by addressing underlying metabolic dysfunction rather than isolated symptoms. However, practical challenges remain:
- Cost and access: Similar therapies cost hundreds to over a thousand dollars monthly without insurance
- Coverage criteria: Insurance likely will require documented MASH with significant fibrosis and failed lifestyle interventions
- Long-term commitment: Potentially lifelong treatment with injectable medication
- Real-world adherence: Clinical trial conditions differ from routine practice
Conclusion
Retatrutide represents a potential paradigm shift in MASLD treatment. The magnitude of liver fat reduction and histological improvement exceeds previous pharmacotherapies, addressing not just symptoms but the interconnected metabolic pathways driving disease progression.
Critical questions about long-term safety, cardiovascular outcomes, and clinical endpoints like liver-related mortality require larger, longer trials. Regulatory approval timelines remain uncertain, with Phase 3 data expected through 2025-2026.
Regardless of whether retatrutide becomes standard of care or catalyzes development of even more effective therapies, the landscape is changing. MASLD patients may soon have evidence-based pharmaceutical options that meaningfully alter disease trajectory.
Frequently Asked Questions
Clinical trials showed measurable reductions within 12 weeks, with continued improvement through 48 weeks. Peak effects typically occurred between 24-48 weeks, though individual responses varied based on baseline metabolic status.
Phase 2 data suggests potential fibrosis regression in some patients, not just prevention of progression. A meaningful percentage showed improved fibrosis stage on biopsy, though more research is needed to identify which patients benefit most.
Gastrointestinal symptoms, nausea, diarrhea, occasional vomiting, affect most patients initially but typically improve after several weeks. Dose titration helps minimize these effects. Serious adverse events remained uncommon in trials, though long-term safety data continues accumulating.
Complete contraindication data is still emerging. Patients with personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2 should avoid GLP-1 receptor agonists. Those with severe gastrointestinal disease, pancreatitis history, or advanced kidney disease require careful evaluation.
Related Topics
Evidence-based articles to support your journey toward sustainable health.
Register your interest in Retatrutide
Retatrutide is currently not available, but once it is approved, you’ll be the first to get notified. Sign up now and stay informed.
Simple one-time sign-up
Early access to availability updates
Exclusive status among the first users
Advantage over non-registered users
%201.webp)
.svg)
.webp)


